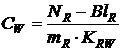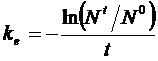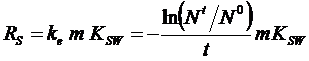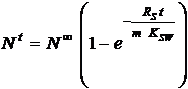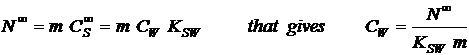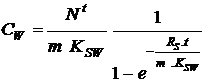Protocol for Participants Yellow shaded text has been added or adapted since birth
Passive Sampling
ICES Trial Survey for hydrophobic organic contaminants in Water and Sediment;
Including laboratory intercalibration.
Patrick Roose, Ton van der Zande and Jacek Tronczynski (ICES/MCWG)
1 Short overview
For the Passive Sampling Trial Survey participants deploy samplers in water preferably in association with mussels. A Passive sampler is silicon rubber (PDMS) sheet spiked with Performance Reference Compounds (PRC). One sampler consists in 6 sheets. Deployment is carried out in duplicate and one sampler is analysed by the participating laboratory and one by a reference laboratory. Samplers are spiked with Performance Reference Compounds (PRC) that will be partially released to the environment. To compare the residual concentration of PRC with the initial amount of PRC, the participants will also analyse a sampler that has not been exposed (to determine PRC initial amount).
A similar exercise is done with sediment but the exposure period will take place in the lab. Wet sediment (preferably fine grained) is shaken with in a bottle coated internally with a thin layer of PDMS (silicone rubber). Where possible participants do parallel uptake bioassays with sediment living organisms. Sediment is shaken in duplicate and one bottle is sent to the reference laboratory. For comparing the amounts PRCs before and after exposure a reference bottle is analysed for PRCs without exposure to sediment.
Therefore, the objectives of the trial are to:
· extend the geographical range of the validation of the use of passive samplers in water.
· transfer knowledge of the methods more widely within the ICES community
· to gain experience in the use of passive samplers
· estimate the contribution of the analytical component to total variability
· to gain further information towards the validation of passive samplers in sediment
The exercise is learning for participants as well as coordinators, Make notes of your experiences during the survey, any suggestions that can help to improve or simplify the procedures.
Unless agreed differently with individuals a standard set of materials as described in here will be supplied to all participants for each station sampled. Note that the silicon sheets and the coated bottles should be stored in the freezer until exposure.
2 Passive Sampling in Water.
2.1 Sampler Frame
The sampler frame is made of stainless steel and this frame has a fixing eye that allows the frame to turn around and give flexibility. You can use a shackle or rope through this eye to fix the sampler frame to what ever you have available to hang the sampler frame on. Secure a shackle with a pin, cable strap or stainless steel wire. Knots in ropes can be secured with cable straps and tape. At RIKZ we expose samplers at 1.5 to 2 meters below surface. In exceptional cases, for example if the total water depth is less than 3 meters it is suggested to hang the sampler frame at half depth. In tidal areas the shallowest depth, i.e. low tide, should be considered for this rule of thumb.
2.2 Deployment
Each participant will receive 3 jars alufoil lined lid with 6 sheets each. Note that for this exercise, one” passive sampler” consists in 6 sheets. Until exposure the jars are stored in a freezer.
Two sets of non-sharp tweezers are required for mounting the sheets and a clean working place to sort the sheets on: either in stainless steel, a place covered with pre-baked (450°C) aluminium foil or a large glass Petri. Sampler sheets may stick these surfaces. Sticking is less when the surfaces are wetted with Milli-Q or local water. Make sure the material you use is clean. Sheets are mounted just before exposure and removed from the sampler directly after recovery. Two sheet holders are already mounted in the frame. A video can downloaded here (20 MB) to give an impression how that works.
(You can change the position to create space for a mussel cage). A cable strap is keeping the fixing rod in place. Cut the cable strap and pull out the fixing rod. Take the sheets from the jar with the number that corresponds to the sampler holder number. If sheets cannot be taken from the bottle one by one because they stick together, take them all out on the Petri dish or wire mesh and separate them using tweezers. Then mount the sheets on the holder with the short side upwards. Feed the fixing wire through the holes on the stem and fix it tight with the cable strap. Mount the sheets on all positions and deploy the samplers for 6 weeks or more. The following parameters are to be recorded and reported in the excel data sheet:
- Date and time of deployment;
- Salinity in o/oo
- Water and air temperature;
- Some measure of SPM; SPM content by filtration or Secci disk.
If your conditions during deployment do not allow
you to mount the sheets onboard. you can unfix the sheet holder from the frame
and mount sheets in the lab. Directly place them in a stainless steel container
or if not available, wrap them in prebaked aluminium foil and transport them in
a cool clean container to the deployment station where they are fixed in the
sampler frame. The time between mounting and exposure should be as short as
possible. Make record of the process
2.3 Recovery after deployment
During recovery the same parameters are recorded as at the time of deployment. Depending on the season and place of deployment the recovered sampler can be clean or totally overgrown with whatever organisms. It is suggested to document the recovered situation by taking picture of the recovered sheets. Sheets that are almost clean are first wiped with a soaking wet tissue and subsequently patted dry with tissue and transferred to the alufoil-lined lid jar in which they were delivered. If fouling organisms grow on the sheet they should be scraped of as completely as possible. Further residues can be removed using a very wet scourer. A nylon type (as use in kitchen) without sponge and rinsed with washed with methanol is appropriate. Work on a wet glass surface and for rinsing you can use local water. When that is not available use Milli-Q, but try to limit the amount of water as much as possible. Only use gloves if local water is that contaminated that contact needs to be avoided, otherwise properly washed and rinsed hands contaminate less than gloves do[i]. The cleaning should be done in the shortest time possible, e.g. less than 5 minutes.
Losses are linearly related to the time of the process. It is not necessary to aim for a sheet as clean as new. Document the situation using a camera. Finally the recovered samplers are place back in the storage jar and stored in the dark, and as soon as possible are transferred to a freezer, until analysis or dispatch to the reference laboratory.
Although the weight of the 6 sheets in one sampler is known within certain limits the exact weight must be determined after extraction. The dry weight of the 6 sheets is considered as the sampler or reference mass (m).
3 Passive sampling experiments in Sediment
A draft guideline for sediment equilibrium sampling is available on the web although the information below contains some specific additional suggestions.
3.1 Material
The standard material delivered is 3 one-liter bottles for each sampling station. The bottles have alufoil lined caps and the inside wall is coated with about 300 mg PDMS spiked with PRC. Please store them in the dark and in a freezer until use. Each bottle is engraved with a number. The exact weight of your bottles (without cap) and the coating weight will be listed on the web. Please weigh the bottles (1 mg accuracy) upon arrival (it is advisable to clean the outside before weighing). Do not stick any labels on the bottle as this will affect the weight.
3.2 Exposure
Sediment samples are taken according OSPAR guidelines. At least 3 kg is collected in a container and homogenised as good as possible The container is preferably glass or stainless steel. If you use any plastic, make sure the residence time is as short as possible. For homogenisation water may be added now to support the mixing process. Sub samples are taken for dry weight determinations. A larger sub sample is taken to determine the total concentration of the target compounds in the sediment, as well as the total Organic Carbon content. Two bottles are filled with the sediment up to 50 to 60% and the amount recorded by weighing the bottle with sediment. Fill the bottles using a wide opening stainless steel or glass funnel..
If the sediment is not well fluidized sufficient water to obtain that situation should be added. Ideally this water should be from location, otherwise milli-Q water can be used, Record the weight again. Then purge the bottle with N2 to remove oxygen as much as possible and close the bottle. The bottle is ready to be shaken (100 rpm) for at least 20 days in the dark at a temperature 20°C. If no climate room is available, find a place with a temperature as close as possible to 20°C . Alternatives to shaking are to roll the bottle (30 RPM), or tumble (30 rpm) but a lower degree of equilibrium will be obtained.
3.3 Recovery.
After the equilibration period the bottles are emptied and vigorously shaken with portions of 50-100 ml milli-Q water to remove all sediment. This should be done in the shortest possible time and using the smallest amount of water. Usually 3 times 30 s is sufficient. Then let the bottle drain upside down on a tissue and/or swing the bottle to remove all water as much as possible. Close the bottle and store in freezer until analysis or dispatch to the reference laboratory. After extraction the bottle is dried and weight of the bottle determined, With the empty weight for the bottle number obtained from the web the weight of the film can be calculated. Comparison with the original weight will show possible wearing during shaking.
4 Analyses of, Bottles and Sheets
4.1 Extraction.
Extraction and cleanup possibilities are described more extensively in the “Draft guidelines for equilibrium passive sampling of sediments”. Many variations are thinkable, some are summarised below: It is important to have the sheets or bottles as dry as possible before extraction. Recovery Standards can be added to the extraction solvent before extraction starts.
Bottles
Before bottles are extracted make sure to swing out the water as much as possible. The bottle can be extracted twice with 50 ml methanol or methanol+acetonitril (1+3 v/v) for 4 hours. After addition of the solvent first acclimatise the bottle to allow the solvent to saturate the vapour phase. Only then close the bottle completely. It is even advisable to warm the bottle slightly under warm water so only pressure reduction can occur in the bottle during the further processes (weighing before and after is a good QC measure). When shaking (horizontal) make sure the solvent is wetting the whole film surface. Otherwise turn the bottle through 180 degrees half way the extraction. An alternative to shaking and safer approach to possible leakage is to roll the bottle for extraction for the same time. A procedural blank is done equally without film in a clean one liter bottle.
Sheets
The simplest and safest way to extract the 6 sheets is putting them loosely in a soxhlet and extract with methanol for 8 hours. (Alternatively methanol+acetonitril (1+3 v/v) can be used). If all sheets do not fit in at once extractions can be done in portions by replacing the extracted sheets after 8 hours and continue with the next portion using the same portion of solvent. A procedural blank is done in the same way, but without sheets.
Another method to extract sheets is cold extraction procedure. Take a 300 or 500 ml Erlenmeyer flask with glass stopper and transfer sheets to it. Add 150 ml methanol and shake gently overnight. Pour out the methanol and repeat the extraction with fresh solvent for another 8 hours. The combined extract is your sample to analyse. It is suggested that completeness of extraction is confirmed by taking all sheets from the different samplings together and extract them once more analysing the extract separately (the most critical compounds here are the higher PCBs). A procedural blank is done in the same way, but without sheets.
Extraction with other solvents is also possible but causes in often considerable swelling (up to 200%) of the sheets and, although not likely after the extensive pre-extraction, the co-extraction of small amounts of oligomers cannot be excluded.
Optional cleanup
Optional is a cleanup with C18 Bounded Silica. This ensures that no oligomers will be present in the extract. For this to be done the extract has to be made into methanol or acetonitrile. Concentrate the extract obtained above to <2 ml. Pre-rinse a column containing 300-500mg C18 bounded silica with 6-10 ml methanol/acetonitrile. Transfer the extract to the column and elute with 6-10ml methanol/acetonitrile. Coronene is the last eluting compound.
Acetonitrile boils at 85°C. Addition of 20% methanol will decrease the boiling point to 64°C. Acetonitrile also forms an azeotrope with water, and so extracts are always dry after boiling down to concentrate.
4.2 Solvent transfers
(1) Azeotropic solvent transfer of methanol/acetonitrile to hexane can be done by concentrating the extract obtained as above to 2 ml and then adding 10 ml hexane for each ml of methanol/acetonitrile. With boiling stones boil the (two phase) mixture down to <2 ml on a water bath. If two phases are still present, repeat the procedure. If the two phases remain it is probably not methanol but water. Add 20 ml hexane and after vortexing 1 minute remove the water with a Pasteur pipette. Evaporate again (Note, this isotropic phase transfer does not work in nitrogen blow down systems). For rota-vapour systems, do not apply vacuum, as the azeotrope will change in an unknown way.
(2) Less efficient is to concentrate the methanol to <50 ml, transfer the extract to a separation funnel with 100 ml hexane or DCM and dilute by addition of mili-Q water until the aqueous phase has less than 20% methanol. Then extract the aqueous phase and repeat this with a second portion of Hexane or DCM. A mixture that has a density of more than 1 g/ml is also suitable. If the organic layer is on the top, the emulsion can be broken, after removal of the separated water phase, by dropping some methanol on it. Evaporation will end in a hexane extract.
4.3 Cleanup
Cleanup of the extract can be carried out according to the laboratory methods used routinely in participant labs. The extracts above are suitable to use in any cleanup you will have available for water, sediment or biota. For sediment extracts from the coated bottles should be treated for sulphur removal.
4.4 Quantification
The target compounds will be quantified as you normally perform analyses of sediments or biota. For determination of the PRCs the qualitative standard delivered can be used for finding the retention times and to set a response factor.
The qualitative standard is dissolved in a small disc of silicon rubber. The PRC s and its amounts will be listed on the web from 25 September, as well retention information. The standard can be dissolved by addition of at least 1 ml of solvent of your choice.
Note that the true concentration of PRCs is not relevant but the ratio between the amount after and before exposure, i.e. sample and reference. Therefore the qualitative standard supplied would be sufficient for calibration. The reference sheet and bottle can also function as a storage blank for non-PRC compounds. This blank should not be subtracted from the sample. Only the procedural blank, i.e. solvent passing through the procedure, is generally subtracted from the sample (and reference) result. However, for this exercise we ask you not to apply blank correction and to report Sample, Reference and Procedural blank data without correction.
4.5 Reporting
The reporting is done on a self-containing spreadsheet that is named according to the matrix and station code. Each field shows the required help information when selected. Where applicable a dropdown menu allows selection of the relevant code. All info on deployment and recovery can be collected on the form. The amounts of target compounds determined on the sheets are reported in ng absolute. Likewise PRC data are reported in amount although peak area/height after correction IS will also apply.
5 Parallel work
5.1 Mussels
Mussels from the sampling location are best depurated for 24 hours using local water. Native mussels or mussels deployed during the water sampling are processed according to the procedures routinely used in your laboratory. Average shelve length should be recorded as well as the average body weight. (The mussels used for the RIKZ mussel watch are selected on a shelve length of ± 50 mm.).
In case additional information on procedures is required an example can be supplied.
Data are reported on a dry weight basis in the same spreadsheet as the water sampling data.
5.2 Sediment assays
Participants are invited to apply uptake bioassays with the sampled sediments using sediment living organisms. Since this will be different among the participants data can be reported in a free format.
5.3 Other contributions
Participants that measure compounds in additional to the target compounds can create more lines in the report sheet. Other additional work such as applying different sampling techniques can be reported in free format if it does not fit in the standard format.
6 Calculation
6.1 Sediment
Calculation of the free dissolved concentration (CW)
in an equilibrated system is done by:
|
|
(1) |
In which NR is the amount (ng) of compound measured in the extract of the sheet/bottle; BlR the procedural (solvent) blank (ng); mR the mass of passive sampling material (kg) after exposure and KRW the material-water partition coefficient (l/kg). The obtained result is in ng/l but it is often more conveniently to express the results on pg/l.
The KRW values are already measured for this material. Presently they are verified by a repeating the measurements. The will be available mid November.
For process QA and further information check the draft guidelines.
6.2 Water
The procedure below is a rule of thumb procedure. More extensive and statistically based procedures are given in literature.
6.2.1 Step 1
Prior to calculating the sampling rate first the PRC data are screened. If the PRC-amount measured is less than 10 times the DL the PRC is rejected. If the amount is more than half the amount of the reference the PRC is also rejected. The remaining compounds are used to determine a sampling rate: RS.
6.2.2 Step 2
The sampling rate can be calculated from the release of the
PRCs that were spiked on the sampler before exposure. The release of compounds
from the passive sampler (PS) follows:
|
|
(2) |
Where N0
is the mass of PRC measured in reference samplers that were not deployed, Nt is the mass of PRC
remaining in the PS after deployment, ke
(d-1) is the first order dissipation constant that rules the release
process, and t the sampling time (d).
After rewriting ke is
calculated from:
|
|
(3) |
From eq. 3, the mass of the sampler (m) (kg) and the KSW
([ii])
(kg/l) the sampling rate RS
(l/d) is calculated through:
|
|
(4) |
The RS values are calculated for all the PRCs
that pass the criteria in step 1. From the obtained RS values the
median is used for further calculation of aqueous phase concentrations.
6.2.3 Step 3
For estimation of the freely dissolved concentration (CW) in the water phase the
full uptake model that is valid for equilibrium and non-equilibrium situations
is applied. The uptake is described by the following equation that includes the
sampling rate (RS) estimate for that specific station and sampling
period in the previous step:
|
|
(5) |
Here Nt
is the amount of compound (ng) in the sampler after deployment for time t
(days). The final amount taken up in the equilibrium situation (N∞) equal the
equilibrium concentration CS∞
times the mass of the sampler (m) in kg. CS∞
is related to CW by the
partition coefficient KSW (l/kg)and consequently:
|
|
(6) |
From eq. 5 and 6 the concentration in the water (CW) in ng/l, is given by:
|
|
(7) |
In equilibrium the first term is dominating and far from equilibrium the second.
6.2.4 Step 4
Report for each sample station a list of the RS values, the median RS in l/d, and CW values in pg/l you have calculated for the target compounds. Any format you like as long as the name abbreviations of the compounds as displayed on the web are used.